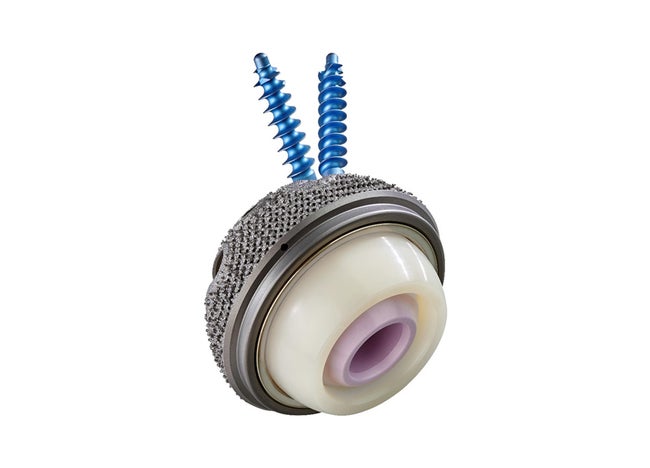
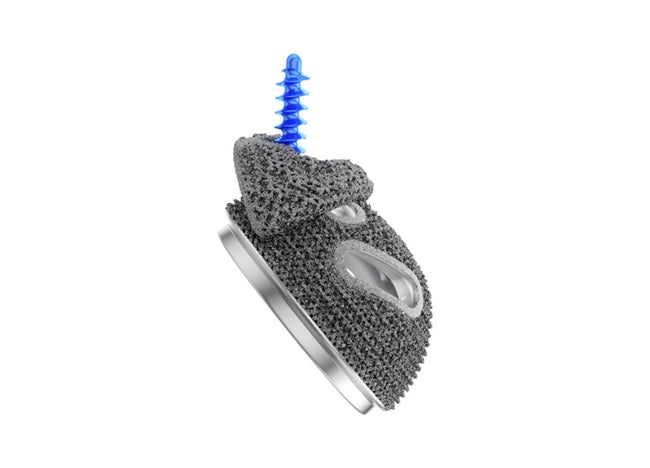
Plasmafit® Revision
Your solution within the Plasmafit® Family
Cementless acetabular revision
Plasmafit® Revision is an acetabular cup system for both primary treatment and revisions of differently located acetabular defect situations.
The hemispherical shape and the laser-sintered titanium structure of the acetabular cup provide high primary stability. To achieve good stability for larger acetabular defects, the design has a total of five options for anchoring screws.
In addition, Structan® Augments can be combined with the Plasmafit® Revision cup for treating larger defects.
Plasmafit® Revision – Characteristics
- High grip thanks to very rough, laser-sintered titanium surface
- Cup design offering several options for screw anchoring
- Two cranial oblong holes for greater flexibility of screw fixation
- The hex connection offers rotational stability during cup implantation
- Roughened surface of inner cone for rotationally stable liner fixation
Plasmafit® Revision – Combinations
- Vitelene® highly crosslinked polyethylene liners with vitamin E
- Six liner options for individual fitting solutions
- Free 360° positioning of the liners
- Fixation screws ø 6.5 mm
- Combination with Structan® Augments
Plasmafit® Revision – Highlight Topics
Laser-Sintering Technology
The profile structure of the Plasmafit® Revision cup surface is characterized by a special, very rough Structan® titanium structure. The structure is produced by an additive 3D printing process. This laser-sintering process permits precise and continuous shaping of the porous and dense implant design structures.
Intraoperative Flexibility
Plasmafit® Revision permits an intraoperative selection of modular liners made of Vitelene® as well as Dual Mobility liners.
Plasmafit® Revision has three single-hole screw holes and two oblong holes. To bridge larger defects, an acetabular Structan® Augment can be additionally implanted.
One Acetabular Cup System
The AESCULAP® Plasmafit® Family provides an acetabular solution for total hip joint replacements. It covers indications from primary up to revision treatments. Patient-specific requirements are addressed in one system and complement each other using the same instruments, inlays, design parameters and surgical procedure.
Learn more about
Plasmafit® Revision with Plasmafit® Dual Mobility
The Plasmafit® Dual Mobility concept addresses a high stability preventing hip joint dislocations.
Plasmafit® Dual Mobility liners can be combined with all cup options of the Plasmafit® Plus and Plasmafit® Revision cup implants. Dual Mobility combination is possible starting with cup size ø 46 mm.
Plasmafit® Revision Liner Anchoring
The design of the Plasmafit® Revision inner geometry permits an intraoperative selection of modular liners made of Vitelene® as well as Dual Mobility liners.
Large-area conical fixation in the cone area of the cup is used to anchor the Plasmafit® Revision liners. The rough titanium inner surface reduces relative movements of the liner to a few micrometers. The conical fixation surface area of the Plasmafit® Revision polyethylene liners also forms a seal against the migration of polyethylene particles from the articulating joint, thus reducing the risk of an osteolysis adjacent to the screw holes.
The Vitelene® Revision liners have a special geometrical shape with a snap connection. This enables mechanical fixation in the Plasmafit® Revision cup. The snap mechanism consists of the protruding snap lip at the upper end of the conical area of the liner and a small gap. The geometry of the inner cup reflects this protruding rim. This snap connection provides the revision liner with an additional anchoring option. The small gap / recess at the cup entrance plane is used to remove the liner in case of a revision.
Plasmafit® Revision with Structan® Augments
Bone defects can exceed the shape and size of the dimension of the revision cup. For stable anchoring and to bridge larger defects, an acetabular Structan® Augment can be additionally implanted.
Structan® Augments consist of a titanium alloy and permit the filling of defects, providing a stable grid structure and high surface roughness. The augments are adapted to the diameter of the Plasmafit® Revision cup. The diameter of the revision cup should be within a range of ±4 mm of the size of the selected Structan® Augments.
Surgical Video
-
Plasmafit® Revision & Structan® Augments
Acetabular Reconstructions
Videos
-
Plasmafit® Revision Acetabular Revision Solutions
Video
-
Plasmafit® Revision & Structan® Augments
Surgical Video