Is poor instrument management messing up your OR scheduling?
Why surgical instruments play a key role for a smooth workflow in the operating room
The schedule in operating rooms (OR) is incredibly tight and adhering to this schedule is a huge challenge. A defined number of Key Performance Indicators (KPIs) have to be achieved each month, e.g. 100 hip surgeries plus 80 knee surgeries. The OR manager is responsible for the adherence to the schedule and the successful in-time performance of the KPIs. Reasons for falling behind schedule include patient behavior, waiting for the entire surgical team to be present, and missing equipment.
In fact, delays were found to be the most common type of error in the OR, contributing to roughly 34 % of all errors. [1] When a delay occurs early in the day, this often leads to subsequent delays throughout the day, as the schedule is so tight that no room is left to catch up – this creates a vicious cycle.
The OR unit: cost-intensive and highly productive at once
The OR unit is a highly productive area and the most cost-intensive unit in a hospital at the same time. This leads in consequence to the fact that any delay adds up to a poorer outcome in KPIs and reduces productivity, including an insufficient room utilization.
Additionally, delays make it more difficult for you to ensure patient safety and optimal surgery outcome. High efficiency would be key to fulfill the expectations of patients and the satisfaction of surgeons and staff.
Clearly, not all delaying factors can be controlled by an OR manager. To ensure successful operations and reduce the stress level for the staff involved, it is essential to focus on those aspects within their reach. Among the preventable delays in question are aspects such as proper scheduling, sterility of surgical sets, instrument condition, instrument availability and sterile good supply process. [2]
What are the main reasons for delays and cancelations in the OR?
Missing and malfunctioning surgical instruments
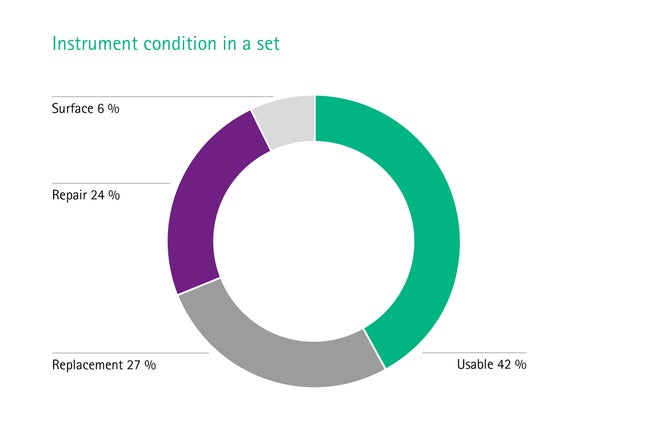
Equipment failure is one of the most common causes of delay, including missing or malfunctioning instruments. This contributes to more than half of all delays in the OR. [1] On average, only 42 % of the instruments in a set are fit for use. The rest needs replacement (27 %), repair (24 %) or surface treatment (6 %) due to corrosive effects. [3], [4] Mostly this failure is based on a bad infrastructure. This includes major factors such as a poorly organized or a non-existent backup stock and not standardized repair routines. Sometimes even an insufficient education of the staff could be the reason.
Replacements during OR procedures is time consuming
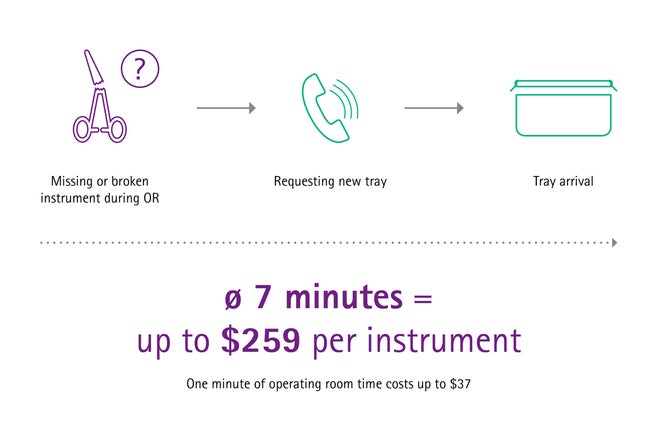
When an instrument is missing or needs to be replaced during a surgery, it takes seven minutes approximately until it arrives in the OR with a new tray. [5] This time-consuming step means a significant time loss and may be risky for the patients’ safety, ruins the schedule and, not least, is costly.
Overloaded surgical sets
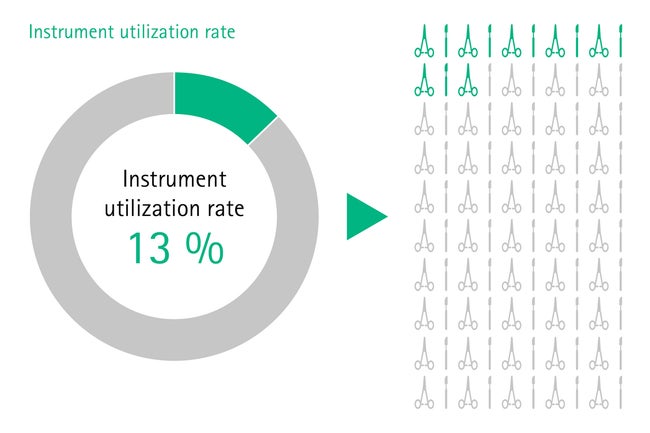
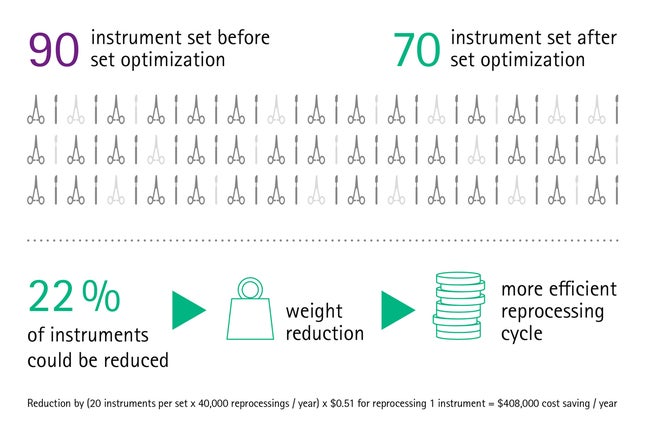
In some hospitals, one set can contain up to 120 surgical instruments and often not half of them are used during surgery. [5], [6] Those sets are most often unstandardized and therefore overloaded, heavy and difficult to handle for the staff in the operating room. Also, the rate of missing or broken instruments increases with the number of instruments per set, [5] leading to even more workflow interruptions.
Incorrectly packed or incomplete sets
Incorrectly packed or incomplete sets do not include what is needed by the surgeon, or even contain the wrong instruments. This might even apply to overloaded sets. For example, gynecological instruments are sometimes packed by mistake into trays for orthopedic operations. [7] Such mistakes can happen quickly when instrument management is not optimal.
Soft wrap packaging is prone to pinholes
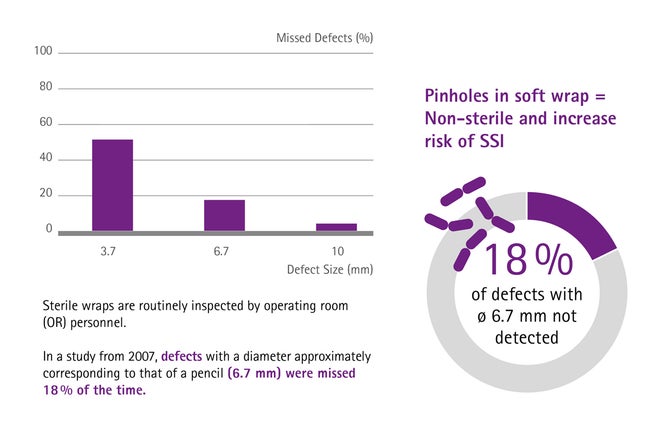
Often, soft wrap packaging is chosen to pack the instruments. The material unfortunately is prone to pinholes, especially if sterilization wraps are not handled appropriately, for example during stocking. When this happens and pinholes are detected in the OR, the whole set is considered as non-sterile and a new set needs to be requested. Disregarding the time needed for a replacement, which adds up to an unnecessary workflow, 18 % of pinholes are not detected by the medical personnel. [8] Regarding the patient safety this may increase the risk of surgical site infections (SSIs).
Communication routines with staff
Even with fully optimized workflows, the handling, transport and delivery of sterile instruments ultimately relies on humans. Thus, good communication between CSSD and OR staff plays a major role for successful surgeries and OR management in general. Vice versa, miscommunication and misunderstandings are at the very least a nuisance – but can at worst have serious consequences.
Increasing or efficiency due to good instrument management
What could be different in your OR management?
Wouldn’t it be an excellent work experience, if you could reduce downtimes significantly and make planning much easier? Naturally, there are circumstances in which you, the OR manager, are entirely powerless. There is nothing you can do if the patient or the surgeon does not turn up or there are difficulties with anesthesia. But time is often lost due to missing or malfunctioning equipment. Surgical instruments in particular tend to be a problem. [3], [4] The entire OR schedule heavily depends on a reliable supply of well-functioning and usable instruments. A highly efficient management of the sterile instrument supply is therefore nothing short of essential – and this is where something can be done, and where we can support you and your Central Sterile Supply Department (CSSD).
At AESCULAP®, we understand the daily challenges an OR manager faces when demanding operating room schedules need to be met. Our teams, with more than a century of industry experience and cooperations with clinics worldwide, are dedicated to help hospitals optimizing their perioperative performance.
Discover your opportunities for improvements
How can efficiency in the OR be increased?
Set optimization
Our findings show that set optimization is a highly important and effective measure: Surgical sets are often overloaded or loaded incorrectly. But sets can be optimized and adapted for a variety of surgical procedures, [9] ensuring that the surgeon has a set containing everything that is needed, but nothing that is unnecessary. This prevents long searches for the required instrument or having to order missing instruments, thus making the whole surgical process fast and efficient.
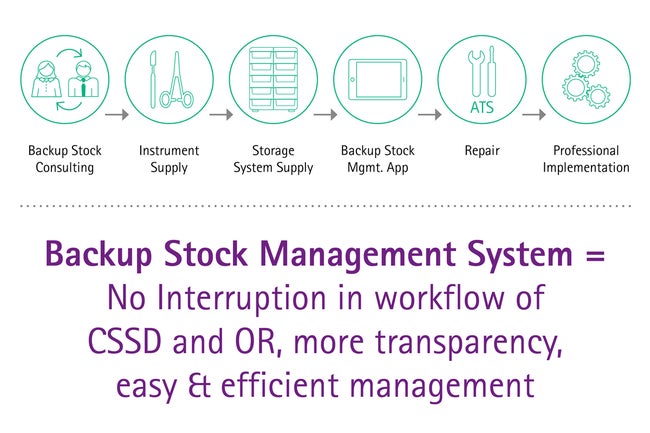
Backup stock management
Building a backup stock based on surgical needs and routines allows a consistent replacement of instruments, for example for instruments under repair or items which have to be reordered. The next time the set is needed the CSSD will still provide a complete assortment with all critical surgical instruments. A backup stock allows repair routines and reorderings without downgrading the set quality or set completeness. Keeping the noteworthy time loss due to reorderings during OR procedures in mind, a backup stock in combination with professional technical service and reordering management is a powerful tool to minimize equipment based OR interruptions.
The Instrument-Mix of the Backup Stock is based on a 4-factor-recommendation developed at AESCULAP®, considering standardized sets contents, wear and tear of items, tray flows and repair management times.
Maintenance and repair
Ensuring the optimal functionality and availability of surgical instruments allows operations to go ahead as planned, avoiding delays, stress and costs. Professional, high-quality repair management and a reliable technical service, that is always available when needed, can help you to achieve the required functionality of the instruments.
Combined with a good backup stock management such a maintenance program creates the basis that functioning surgical devices are always available. This allows operations to go ahead as planned with the right set in the right place at the right time and in the right condition.
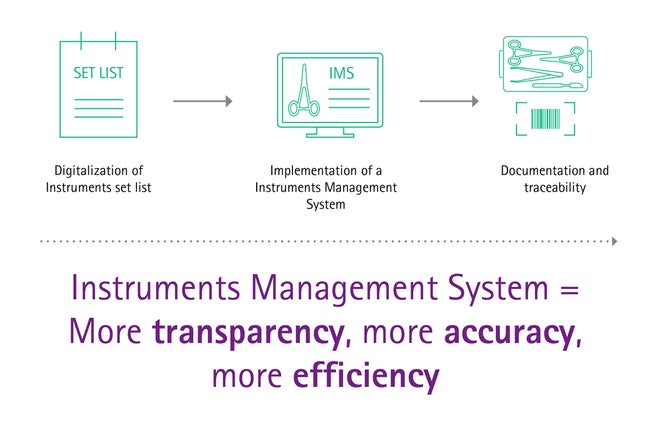
Digitalization and instrument management system
The global trend of digitalization is a driver of automation and efficiency. Recent advances made in these areas can also be of benefit for surgical asset management. A study showed that most hazards connected to the delivery of instruments can be overcome using IT supported systems (Instrument Management Software, IMS). [10]
Modern digital solutions reduce assembling errors and workload in an easy and smart way.
To give you an example: Standardized items lists for trays, where inserted items can simply be ticked off, avoid mishaps during packing. Solutions like that avoid a remarkable number of delays during OP procedures and help you keeping track of your daily schedule. More holistic digital solutions even contribute to cost transparency, analytics or legal documentation.
Containerization
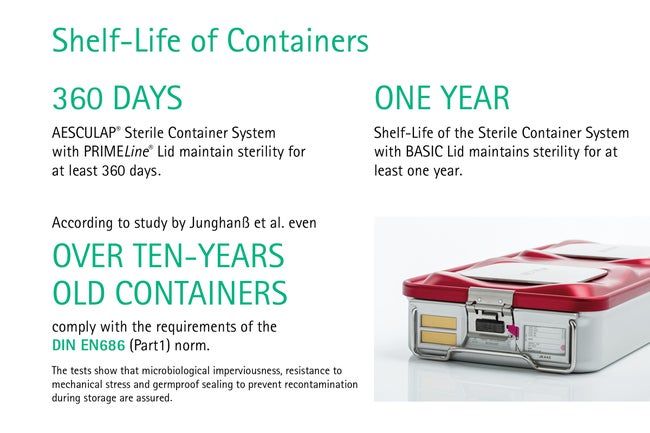
Soft wraps are commonly used to pack sterilized surgical instruments but are very prone to pinholes. Damaged packaging cannot keep the contents sterile and is a high risk for the patient’s health. In contrast, containerization does not only enable safer storage, but also allows an easy function control and most importantly, a reliable aseptically presentation. Containers with PrimeLine® Lid maintain sterility for at least 360 days. [11] Shelf-Live of Containers with Basic Lid maintain sterility for at least one year. [12] Over ten-years old containers comply with the requirements of the DIN EN686 (Part1) norm. [13]
Staff training
The supply of sterile instruments relies on proper communication. Without it, no one would know which instruments are needed, let alone when and where. It is essential that the staff is aware of the importance of good communication, as without it the OR cannot run like clockwork. Fortunately, good communication skills and dealing with conflicts can be learned. Well-trained staff will not just make fewer mistakes and identify possible hazards faster, but also actively contribute to improving the entire process.
A well-structured workflow in the CSSD and the OR as well as high-quality surgical instruments are essential for performing successful surgeries, ensuring a better patient safety, and increasing the number of treatable patients. Establishing smooth working routines prevents conflicts and errors and makes processes more efficient.
With modular solutions, adapting to yearly budgets or specific needs, virtually every hospital has access to the goal of providing the right set at the right place and the right time in right condition. We provide an in-depth analysis of your unit and based on our findings we can show you how to avoid preventable downtime.
[1] Wong, J., et al. Delays in the operating room: signs of an imperfect system. Canadian Journal of Surgery, 2010. 53(3): 189.
[2] Ford, S. Exclusive: Stress levels at work making nurses ill, finds survey. 2014
[3] Aesculap. Data on file.
[4] Lunardini D, Arington R, Canacari EG, et al. Lean principles to optimize instrument utilization for spine surgery in an academic medical center: an opportunity to standardize, cut costs, and build a culture of improvement. Spine 2014;39(20):1714-17.
[5] Stockert EW, Langerman A. Assessing the magnitude and costs of intraoperative inefficiencies attributable to surgical instrument trays. J Am Coll Surg 2014;219(4):646-55.
[6] Paula JRdA, Silva RdCRd, Vedovato CA, et al. Instrumentais nas caixas cirúrgicas: avaliação de custo. Rev. Sobecc 2015;20(2):73-80.
[7] Jeffreys B (2008). NHS ‘chaos’ over surgical tools. BBC, 24 April 2008.
[8] Waked WR, Simpson AK, Miller CP, et al. Sterilization wrap inspections do not adequately evaluate instrument sterility. Clin Orthop Relat Res 2007;462:207-11.
[9] Yoon, S., et al., Implementation and Impact of a Hospital-wide Instrument Set Review: Early Experiences at a Multisite Tertiary Care Academic Institution. American Journal of Medical Quality, 2019. 34(1):67-73
[10] Guédon, A.C., et al., Where are my instruments? Hazards in delivery of surgical instruments. Surgical endoscopy, 2016. 30(7):2728-2735
[11] Aesculap Sterile Technologies, Real time Event Related 360-day Shelf Life Study: STERILCONTAINER system with PrimeLine Lid, DOC570 Rev. B 3M 9/06.
[12] Aesculap, Sterilization Validation Study, One year Shelf-Life of the STERILCONATINERTM system with BASIC Lid, DOC 173 REV 5M 1/02.
[13] Junghanß U., Winterfeld S., Gabele L., Kulow U., Hygienisch-mikrobiologische und technische Überprüfung von Sterilisier-Containersystemen, Zentralsterilisation 1999, 7 (3):154-162.