Hidden costs due to poor Instrument Management
Unclear costs of a new hospital or CSSD getting you down?
As a Health Care Director or Project Manager of a new hospital or new CSSD, one of the most important tasks in the Equipment Planning process is to determine the right surgical sets and the corresponding safe sterile packaging system for meeting the future demands in the Operating Room.
But, planning of surgical sets is not as easy as one may have thought: It needs to be defined HOW MANY SETS of WHICH DISCIPLINE with WHICH INSTRUMENTS inside will be required.
Common mistakes in sterile goods planning
Why repeating the mistakes of the past and increasing the investment costs?
Impact of copying and pasting
One common mistake in the equipment planning process is the practice of “copying and pasting” surgical set structures, set contents and sterile packaging system from already existing healthcare facilities. Unfortunately, very often these set lists from existing hospitals do not match with the new facility’s future OR demands and thereby are not useful. In addition to that, many sets of already existing hospitals are inefficient in terms of structures and contents. [1]
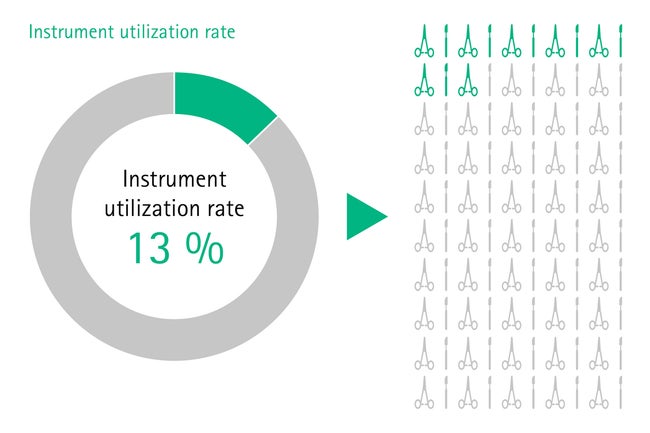
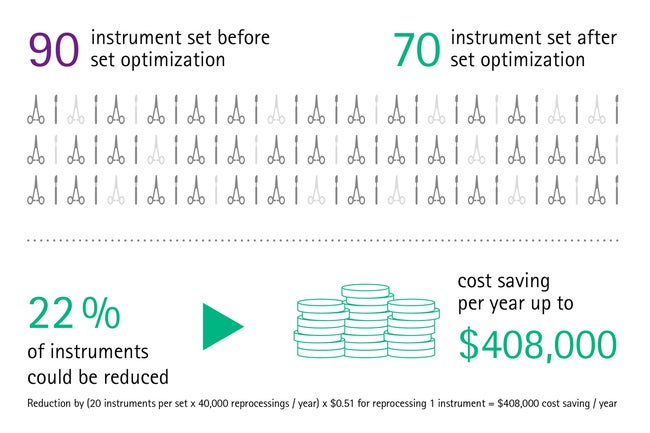
For instance, many instruments within a set are never used during surgery. A study shows that the instrument utilization rate often is as low as 13% due to overloading of sets. [2] After surgery, all devices should be reprocessed, even if they were not used. This causes significant unnecessary costs for health institutions, considering that the total reprocessing costs per instrument exceed US$ 0,51. [3]
Impact of underestimating Total Cost of Ownership (TCO)
In many purchase decisions different offers are compared. When selecting the supplier one decisive factor is the lower price for the offered equipment. [4] Unfortunately, the so called Total Cost of Ownership (TCO), considering the direct but also the indirect costs during the equipment lifetime are not considered in the equipment planning process. An analysis shows that the price for low-end medical devices only makes up approximately between 20-25% of the TCO. [5]
The following example is showing that poor set planning and neglecting of care and maintenance could result in high unnecessary costs:
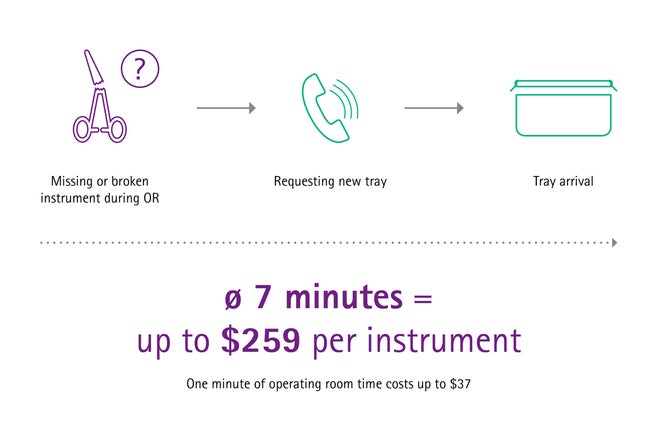
The price of a single missing or malfunctioning surgical instrument during a surgery can be really expensive. When a new tray has to be ordered during surgery, it takes approximately seven minutes until it arrives in the OR. [3] One minute of OR time costs up to US$ 37, [4] which means that one missing or malfunctioning instrument can cost up to US$ 259 only due to the delay it is causing.
Impact of surgical instruments and sterile packaging system in planning a CSSD
The quantity and configuration of surgical sets has also a huge impact in the planning and dimensioning of central sterile supply department (CSSD). For example, by calculating the required floor space of CSSD, it is absolutely necessary to consider: the number of future surgeries, the quantity and features of medical devices and also the sterile packaging system, that needs to be transported, cleaned, disinfected, sterilized and stored. The German Sterile Association advises against calculating the required spaces based on the number of hospital beds, as there is no direct correlation between hospital beds and space needs of the hospital. [6]
Equipment planning for new health care facilities is a complex process where not only clinical process knowledge, but also expertise in sterile goods management, as well as in architectural and capacity planning is required. Additionally investment budget limitations may prevent project managers to invest in the most sustainable sterile goods management solution with the most beneficial TCO. But don’t worry, there are some attractive solutions and concepts supporting you in this process.
Avoid mistaken investment for new hospital or cssd constructions
Planneable costs and professional instrument management planning
Successful equipment planning for new healthcare institutions can be achieved by the right interdisciplinary team and well-coordinated project planning. Generally, a good project team consists of stakeholders with clinical and process knowledge, project managers, architects [7] and experienced Surgical Asset Management consultants who are responsible for planning the CSSD capacities and surgical equipment in order to meet the future OR demands in the best possible way.
Bear in mind, that collaborating with experienced Surgical Asset Management consultants for planning the future set quantities, set structures and sterile packaging solution can pay off and avoid unnecessary investment and running costs. [4]
For optimizing the future Total Cost of Ownership (TCO) of surgical equipment it is recommended to also consider a professional Value Preservation and Backup Stock Management solution, a safe and efficient sterile packaging solution, as well as Digital Tools for process safety and traceability.
Learn more about these solutions:
Set planning
Professional planning of surgical instruments in new construction hospital projects prevents from oversizing surgical inventory, overloading surgical sets and thereby avoids associated extra costs (e.g. unnecessary reprocessing costs).
Experts in set planning support you in defining the right set quantities, set structures and sterile packaging solution to meet your healthcare’s future OR demands. In this process the number of future operating rooms and the projected surgeries in the different disciplines of the future health care institution is taken into account.
This professional approach is also vital for defining the architectural design of the future hospital’s CSSD, as it enables, for example, conclusions regarding the needed number of washer and disinfector machines and sterilizers.
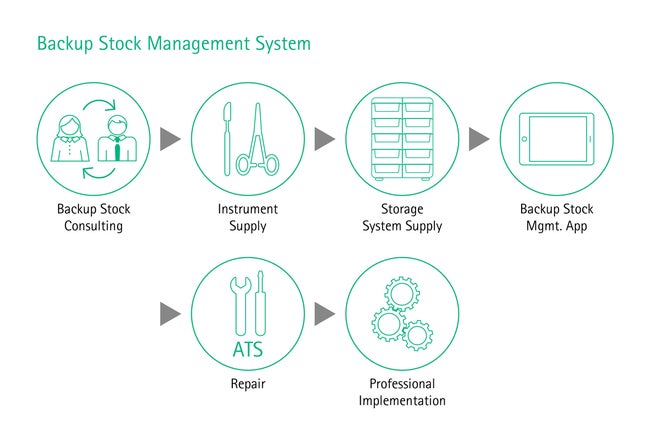
Value preservation & backup stock management
Considering the Total Cost of Ownership in the planning and acquisition process of surgical equipment and sterile goods supply could increase the performance of your future health care facility. [8] Ensuring the optimal functionality and availability of surgical equipment allows operations to go ahead as planned, avoiding delays, stress and costs. A professional high-quality repair management and a technical service that is reliable and always available when needed can help you to achieve a long lifetime and proper functioning of your instruments and thus preserves the value of your surgical sets. Moreover, for guaranteeing that sets are complete with functioning instruments during surgery, a well-defined backup stock where high-runner and critical items are available as a backup, is recommended. This will enable surgeries to go ahead as planned while broken or lost instruments get repaired or replaced.
Digital tools
Sterile packaging solution
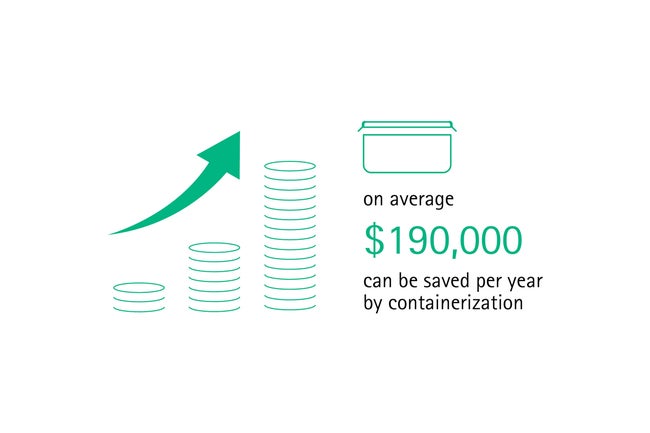
In many markets, soft wraps are commonly used to pack sterilized surgical instruments but are very prone to pinholes or tears. Damaged packaging cannot guarantee sterility of the instruments and is a high risk for the patient’s health. By contrast, containerization does not only enable safer storage, but also easier handling as containers can be stacked and different lid colors allow for sorting by disciplines. Containers prevent contamination of sets and maintain sterility for at least 360 days. [10] One case study found that – compared to soft wrap packaging – on average US$ 190,000 can be saved per year by containerization. [11]
When planning the surgical equipment, the holistic sterile goods management process shall be considered for optimizing the future TCO. Therefore it is recommended to do professional set planning, invest in a backup stock management and value preservation solution, to look for a safe sterile packaging solution and also to support the processes with digital tools. Now one may think that such a comprehensive but sustainable solution requires high investment budgets. But there exist very smart solutions, such as SAM Fleet Care by AESCULAP®, which will go easy with your investment budget.
[1] Emely Walker Stocker, MBA, Alexander Langerman, MD assessing the Magnitude and Costs of Intraoperative Inefficiencies Atributable to Surgical Instrument Trays. https://pubmed.ncbi.nlm.nih.gov/25154669/
[2] Stockert EW, Langerman A. Assessing the magnitude and costs of intraoperative inefficiencies attributable to surgical instrument trays. J Am Coll Surg 2014;219(4):646-55.
[3] Stockert EW, Langerman A. Assessing the magnitude and costs of intraoperative inefficiencies attributable to surgical instrument trays. J Am Coll Surg 2014;219(4):646-55.
[4] Petra Hospodková and Aneta Vochyánová, The application of the total cost of ownership approach to medical equipment – case study in the Czech Republic, https://www.researchgate.net/publication/325452669_The_Application_of_the_Total_Cost_of_Ownership_Approach_to_Medical_Equipment-Case_Study_in_the_Czech_Republic
[5] Total Cost of ownership, https://bmet.fandom.com/wiki/Total_Cost_of_Ownership
[6] Recommendations by the Committee for Hygiene, Construction and Technology Requirements for construction or reconstruction of a Reprocessing Unit for Medical Devices (RUMED). Central Service 4/2014, 264-267.
[7] Ray Bielby, International Health Facility Guidelines, Version 2: May 2017, http://healthfacilityguidelines.com/ViewPDF/ViewIndexPDF/iHFG_part_q_2-equipment_planning
[8] Keith Martinko, How Understanding the Total Cost of Ownership of Your Equipment or Instrumentation Can Reduce Costs, Increase Performance and Improve Workforce Productivity. https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/D19487~.pdf
[9] Xiaolian Zhu†, Lan Yuan†, Tianyi Li and Ping Cheng*,Errors in packaging surgical instruments based on a surgical instrument tracking system: an observational study, BMC Health Services Research (2019) 19:176 https://doi.org/10.1186/s12913-019-4007-3
[10] Aesculap Test results performed with the PrimeLine® PRO lid: The test involved how the PrimeLine® PRO lids behave in combination with the Basic container bottoms. After sterilization of sterile containers the bacterial growth in an event related storage after 30, 180 and 365 days were tested. The validations of the container systems showed no bacterial growth. The biological reliability conforming to EN ISO 10993-18 was evaluated. All test results are on file at Aesculap AG.
[11] Practice Greenhealth. Greening the OR: Reusable Hard Cases for Surgical Instrumentation 2013.